Overview
The obesity epidemic is considered one of the most serious threats to public health in the 21st century, impacting 36% of the United States and 18% of the world’s adult populations (Body Mass Index or BMI over 30). Obesity creates significant health risks for a variety of associated diseases including type 2 diabetes, cardiovascular diseases and even cancers. Indeed, around 9.4% of U.S. and 8.5% of world populations have diabetes. Asian countries including Korea are not safe from obesity epidemics. Though only 5.3% of Koreans have a BMI over 30, alarmingly 14.4% of Korean adults have diabetes due to being overweight and obese. Unfortunately, there are only limited medication and treatment options available to manage patients’ body weight and blood glucose levels over their entire life. Thus, finding new and effective ways to treat obesity and diabetes is critical.
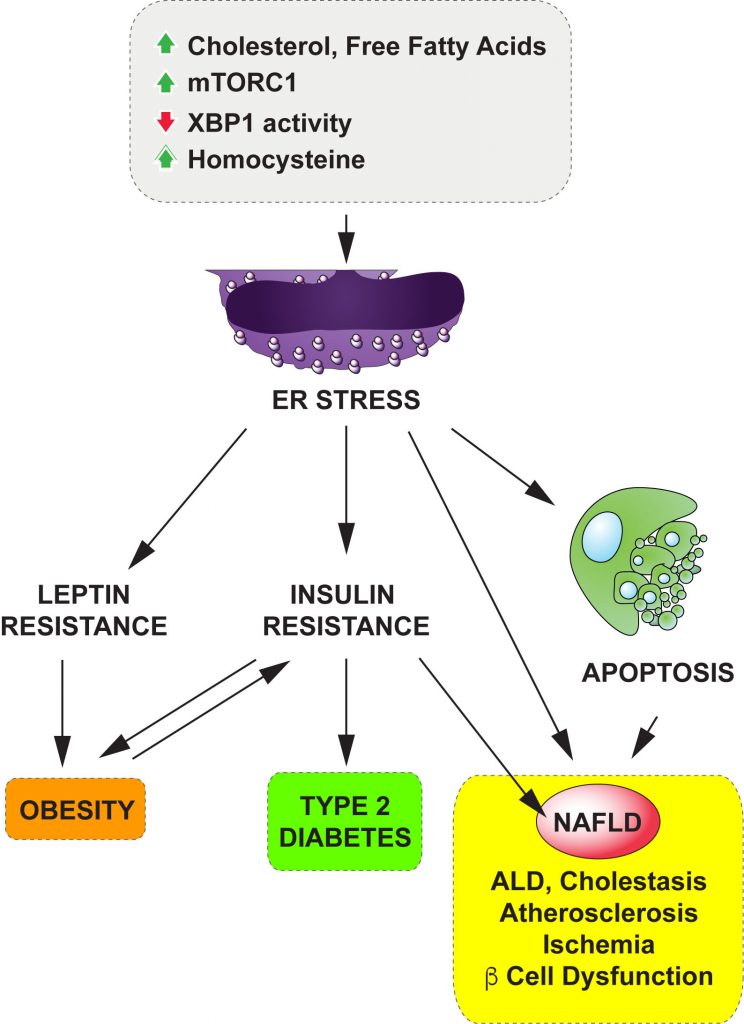
Our body weight is largely determined by the balance between energy intake (through food consumption) and energy expenditure (in the form of exercise or thermogenesis). Leptin, a hormone secreted from adipose (fat) tissue, is a major regulatory factor for both food intake and energy expenditure by acting on the hypothalamus in the brain. Glucose homeostasis is mainly regulated by insulin, a hormone secreted from the pancreas. Insulin increases glucose uptake into the muscle and fat tissues and suppresses new glucose production from the liver. However, obesity leads to leptin and insulin resistance and ultimately type 2 diabetes.
Recent studies have demonstrated that increased endoplasmic reticulum (ER) stress leads to leptin and insulin resistance while interventions to lower ER stress reverse them in obese and diabetic animal models as well as in human patients. These studies strongly suggest that signaling events during ER stress play a pivotal role on various metabolic regulations and can be promising therapeutic targets to treat metabolic diseases such as obesity and diabetes.
Research Summary
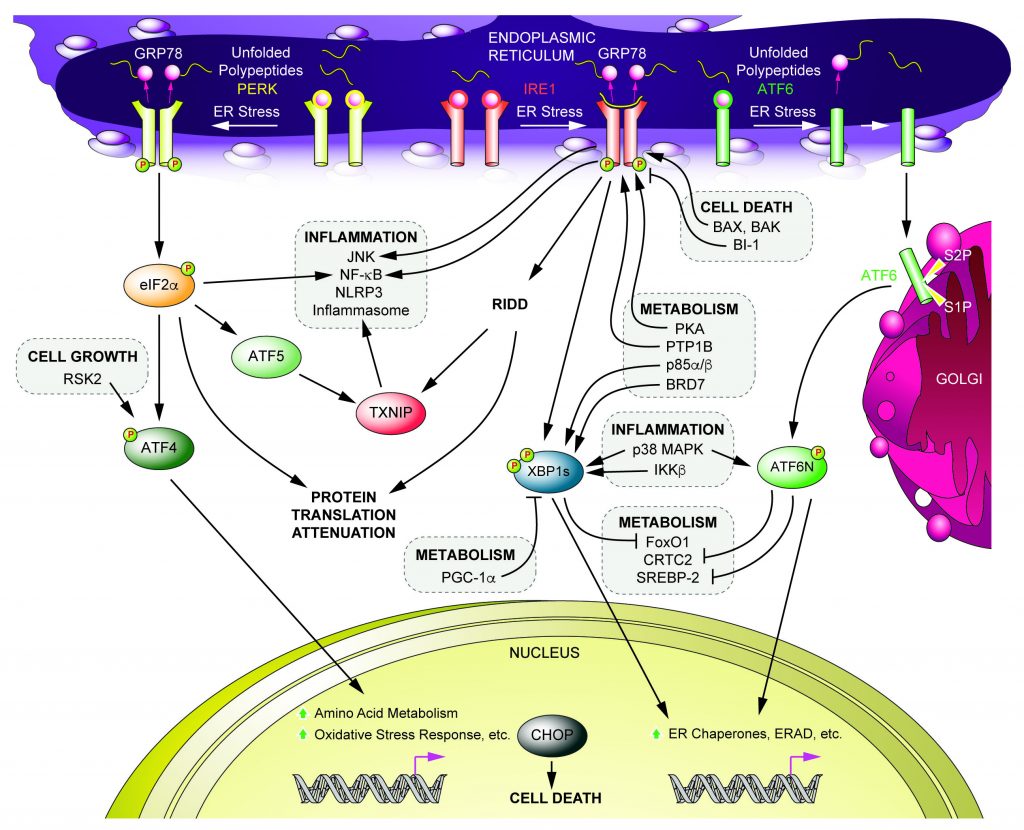
Cells under ER stress employ signaling events called the “unfolded protein response” (UPR) to restore ER homeostasis. Among signaling molecules in UPR, X-box binding protein 1 (XBP1s) is one of key signaling molecules and has been shown to play a crucial role in the hypothalamus, liver and pancreatic β cells’ metabolic regulation. There is “cross-talk” between XBP1s and other signaling molecules including p38 MAPK, IKKβ, Brd7, PI3K, PGC-1α and FoxO1. Furthermore, XBP1s interactome is critically involved in metabolic homeostasis. These findings clearly demonstrate that ER stress and its related signaling pathways are critically important in metabolic regulation.
Our research interests stem from the existence of various interactions surrounding UPR and their critical involvement in metabolic homeostasis, and their subsequent potentials as therapeutics toward obesity, type 2 diabetes and other metabolic disorders. We identify novel interactions between UPR and other signaling pathways and investigate their roles in metabolic homeostasis. Our research currently focuses on the following topics.
- Identification of novel crosstalk between UPR and other signal transduction pathways (UPR interactome)
- Identification of novel post-translational modifications (PTMs) on UPR signaling molecules (such as phosphorylation and ubiquitination)
- Investigation of the physiological role of crosstalk and PTMs of UPR on energy, glucose and lipid metabolism
- Investigation of the pathophysiological role of crosstalk and PTMs of UPR on the development of obesity, type 2 diabetes and other metabolic disorders.